Cat#:Y05801
Name:Recombinant Human EPO(Y05801)
Species:Human
Specification:10μg/50μg/1mg
Purity:≥95%
Endotoxin: ≤10 EU/mg
Expression System:CHO
 Product Brochure
Product Brochure
Cat#:Y05801
Name:Recombinant Human EPO(Y05801)
Species:Human
Specification:10μg/50μg/1mg
Purity:≥95%
Endotoxin: ≤10 EU/mg
Expression System:CHO
Recombinant Human Erythropoietin (EPO, Y05801) is a biologically active cytokine expressed in Chinese Hamster Ovary (CHO) cells, designed to deliver consistent performance in cell proliferation and hematopoietic studies. With a purity of ≥95% (validated by SDS-PAGE under reducing and non-reducing conditions) and endotoxin levels ≤10 EU/mg, this product meets the strict standards required for advanced research and biopharmaceutical manufacturing.
The bioactivity of this recombinant human EPO is confirmed in TF-1 human erythroleukemic cell assays, achieving an ED50 ≤0.01 ng/mL, demonstrating its exceptional potency and reliability. The molecular weight (18.3 kDa) and tag-free sequence (Ala28–Arg193) ensure compatibility in sensitive experimental systems where protein modifications may interfere with results.
Produced through a validated CHO expression system, our recombinant EPO maintains proper glycosylation and folding, essential for biological functionality. Each batch is lyophilized from sterile PBS buffer containing stabilizers (mannitol and Tween 80) for superior stability and reproducibility.
Storage & Stability:
Lyophilized: Stable for 36 months at -20°C to -80°C
After reconstitution: Stable for 6 months (under sterile conditions)
Recommended: Avoid freeze-thaw cycles, use manual defrost freezer
Shipping & Packaging:
Shipped with blue ice to maintain cold chain integrity. Available sizes include 10 μg, 50 μg, and 1 mg per vial, ensuring flexibility for both small-scale academic studies and large-scale industrial applications.
Applications:
Cell proliferation and differentiation assays
Erythroid cell culture and hematopoietic research
Drug screening and biopharmaceutical development
Quality control reference standard for cytokine studies
With over a decade of expertise in recombinant protein manufacturing, our company provides high-quality, research-grade cytokines trusted by pharmaceutical companies and research institutes worldwide. Each lot undergoes rigorous bioactivity validation, purity testing, and endotoxin screening, ensuring consistent performance and reproducible outcomes.
Why Choose Us:
As a professional recombinant protein manufacturer and supplier, we combine scientific expertise, strict quality management, and flexible customization services. Whether you require bulk production or specific formulations, our technical team can support your experimental design and application optimization.
Call to Action:
Contact our scientific support team today for custom EPO protein solutions, technical consultation, or a quotation tailored to your research needs.
SDS-PAGE
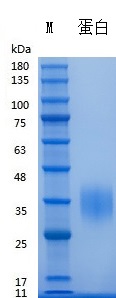
Recombinant Human EPO (Cat. No. Y05801) SDS-PAGE under reducing (R) & Non-reducing conditions. The gel was stained with Y05801 SDS-PAGE.
Bioactivity
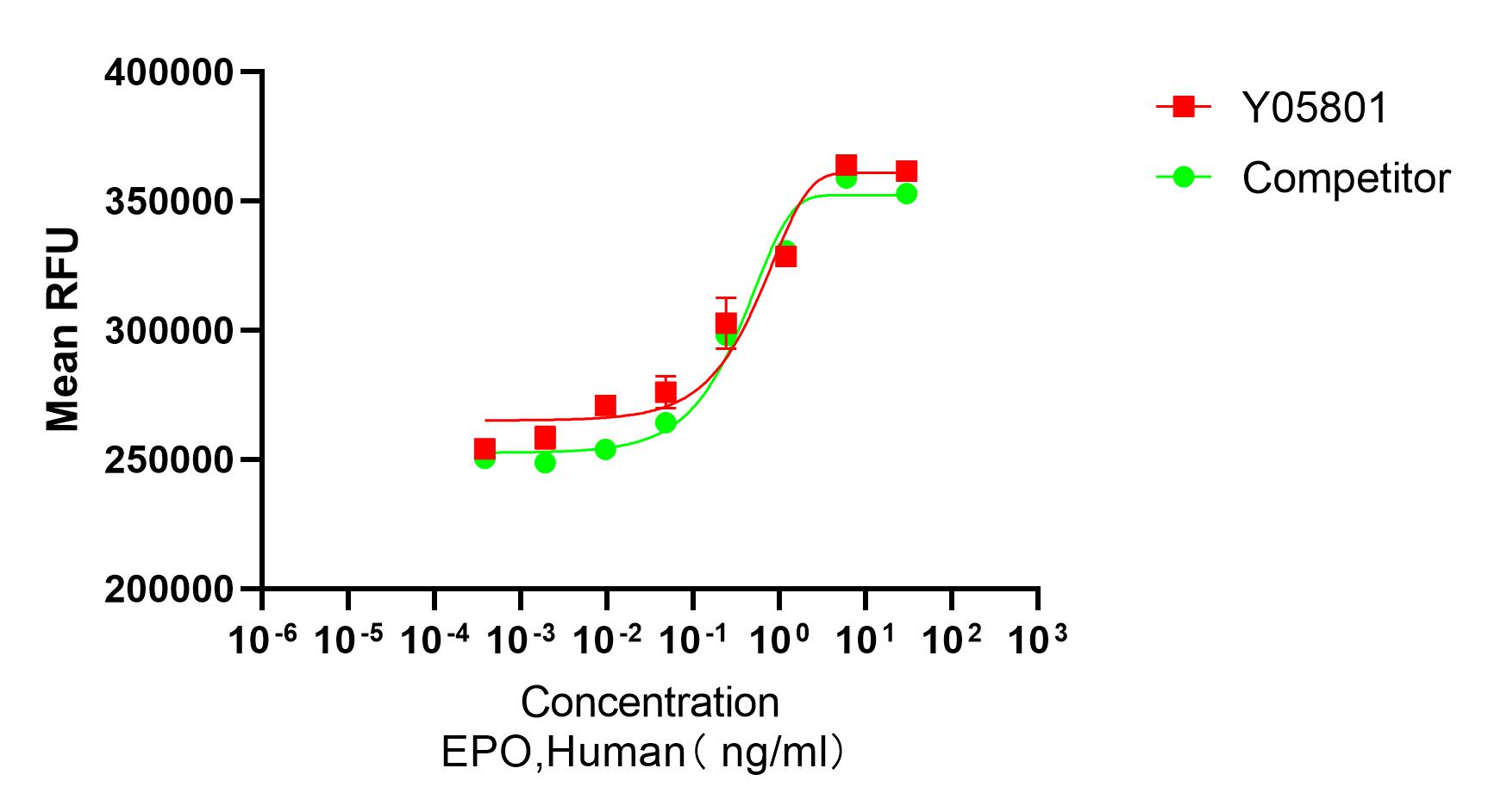
Recombinant Human EPO (Cat. No.Y05801) stimulates proliferation of TF-1 human erythroleukemic cells. The ED50 for this effect is ≤0.01 ng/mL. TheBioactivity of Recombinant Human EPO (Cat. No. Y05801) was higher than the other competing product.

Jiangsu East-Mab Bio:Building 13 and 17, 888 Zhujiang Road, Nantong, Jiangsu 226499 , China
Suzhou East-Mab Bio:Floor 5 & 6, Building 1, 168 Majian Road, Suzhou, Jiangsu 215129, China
Platform Information Submission-Privacy Agreement
· Privacy Policy
No content yet
Platform Information Statement-Laws and Regulations
· Laws and regulations
Trademark registration of Jiangsu Dongkang Biomedical Technology Co., Ltd.
East Mab
East Mab Bio
东抗生物
To download COA, please enter the product batch and specification information in the search box.
You can also ask us directly via the following email: dongkang@Sales.com